- Details
As the ANA turns 40, we want to hear from patients, family members and caregivers. Tell us how the ANA has impacted your AN journey - just a quick snippet about some unique part of your experience or something that made a significant difference for you. Include a photo! Submit your impact story.
- Details
The ANA At A Glance
Together For Better
Together for better care
Together for better outcomes
Together for better discoveries
The Acoustic Neuroma Association, the premier resource to the acoustic neuroma community, informs, educates and supports those affected by acoustic neuroma brain tumors.
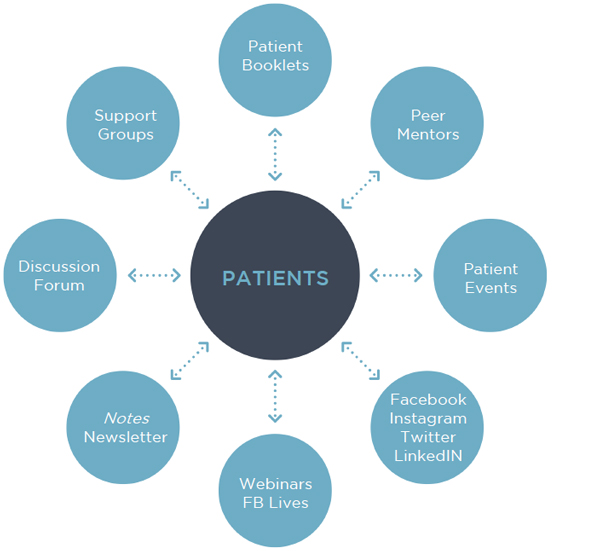
The ANA offers several partnership opportunities for medical providers.
Read more about our Ways to Partner.
- Details
Patient Perspectives in Vestibular Schwannoma Surveillance
You are being invited to participate in a research study titled Patient Perspectives in Vestibular Schwannoma Surveillance. This study is being conducted by Jay Piccirillo, MD, Principal Investigator.
Study Information:
The researchers want to interview patients who are either actively undergoing observation or underwent observation before ultimately receiving treatment for their vestibular schwannoma. They hope that in conducting these interviews they can not only gain insight into the important factors in this decision making process but also help guide future research for patients considering to undergo observation for their vestibular schwannoma.
Am I Eligible to Participate?
Participants are eligible for this study if they are 18 years or older, live in the United States, speak English, have access to a phone for a 30 minute conversation, have chosen to observe their vestibular schwannoma, and have not been diagnosed with NF2.
What Does Participating in This Study Involve?
- Completion of one screening survey (5 minutes)
- Completion of one follow up survey (10 minutes)
- Completion of one phone interview (30 minutes)
Participants who are interested will be asked to fill out an initial screening survey via REDCap, those who are eligible will be instructed to fill out one additional survey as well as be contacted to coordinate an interview time.
As a thank you for helping advance the collective knowledge of this condition, the research team is offering a $20 reimbursement for anyone who completes the interview.
In order to determine if you meet the eligibility criteria for the study please complete the Screening Survey.
If you are eligible, we will contact you with additional questions and coordinate the interview for the study.
If you have questions please email otooutcomes@wustl.edu or call Jay Piccirillo, MD, Principal Investigator, at (314) 362-4356.
- Details

#TheANAis40!!
We are excited to celebrate this milestone in different ways throughout the year.
We hope you will join us as we look back with appreciation and forward with determination.
Calling all artists!
We want to highlight artwork from the AN community to commemorate this momentous occasion. If you are an artist - painter, graphic designer, photographer, sketch artist, whatever! - we would love it if you sent us something to be displayed in our Showcase. Our hope is that by creating a collection of these wonderful pieces, we might tell a great, patient-focused, artistic story for our 40th anniversary. More Information.
 Sample: The sunset bicycle photo symbolizes my joy in recovering bike riding for work commuting as well as for enjoyment. I also really love this quote for the AN journey, "Life is like riding a bicyle: to keep your balance you have to keep moving."
Sample: The sunset bicycle photo symbolizes my joy in recovering bike riding for work commuting as well as for enjoyment. I also really love this quote for the AN journey, "Life is like riding a bicyle: to keep your balance you have to keep moving."
Impact Stories
As the ANA turns 40, we want to hear from patients, family members and caregivers. Tell us how the ANA has impacted your AN journey - just a quick snippet about some unique part of your experience or something that made a significant difference for you. Include a photo if you'd like!
 Sample: These days, I live a busy, active life chasing my two daughters and owning and operating campgrounds. When I was diagnosed six years ago, finding others my age who were also dealing with acoustic neuroma was not easy. The ANA connected me with other young patients who could relate to my situation and then helped me form a support group and a Facebook group for young adults. These groups have allowed me to share my unique experiences and learn from others as they have shared theirs - it's been truly amazing and I am so grateful to the ANA for the help and support I received on my journey. - Emily
Sample: These days, I live a busy, active life chasing my two daughters and owning and operating campgrounds. When I was diagnosed six years ago, finding others my age who were also dealing with acoustic neuroma was not easy. The ANA connected me with other young patients who could relate to my situation and then helped me form a support group and a Facebook group for young adults. These groups have allowed me to share my unique experiences and learn from others as they have shared theirs - it's been truly amazing and I am so grateful to the ANA for the help and support I received on my journey. - Emily
Get Your Gear
Get your special edition anniversary t-shirt and water bottle commemorating 40 years of the ANA. Shop now.
The ANA was founded in Carlisle, PA in 1981 by Virginia Fickel Ehr. After having surgery for the removal of an acoustic neuroma in 1977, she resolved that future acoustic neuroma patients should have easy-to-read medical materials about their condition, as well as support and comfort from other patients.
With the help of her physician, she contacted eight other patients and formed the Acoustic Neuroma Association to provide the information and support to newly diagnosed patients that she was unable to find when she was looking.
We are incredibly grateful to Ginny and the other founding staff, members, and board of directors - we wouldn't be here today without them.
- Details
Research Grant Program
 The Mission
The Mission
The ANA is dedicated to furthering all aspects of scientific knowledge related to acoustic neuromas, including its cause(s), development, and treatment, thus improving the lives of individuals living with AN, and finding its ultimate cure.
Our Interest Areas of Funding
We encourage investigators to submit grant proposals across the broad spectrum of acoustic neuroma. These areas may be original research drawn from disciplines such as audiology, biology, psychology, sociology, or pharmacology.
Our Research Committee has additionally identified three broad areas of interest:
Causes and Cures: This includes such areas as chromosomal, cellular, genetic studies, etc.
Technological, Cultural, and Social Interventions: The use of technology in post-treatment, including health apps in monitoring health conditions, and the effectiveness of online self-directed therapies for depression, fatigue, cognitive skills, physical strength/stability, biofeedback, cochlear implant, etc.
Quality of Life: Consideration given to Pain Management – including headaches – and issues related to facial paralysis, social, emotional, cognitive, etc.
Eligibility
The ANA will consider funding researchers and/or centers who have a formal affiliation with nonprofit, academic, medical, or research institutions. The principal investigator must have an appropriate level of background, training, and institutional engagement to support the proposed research.
Amounts Available for Funding
Individual awards are typically up to $25,000 in a given year. Multi-year projects may be considered if they are consistent with our research initiative. Funding beyond the first year will be on a year-to-year basis, and multi-year projects are subject to annual review for ongoing funding.
Our Annual Grant Cycle
The grant cycle is straightforward: a Request for Proposals (RFP), our review of submissions, and our announcement of grants awarded.
The RFP will typically be announced at least once annually, during the second quarter of the year. Typically, multiple RFPs will have a different mission and may not be made on a predetermined basis. The time from our announcement to our proposal deadline will be of a reasonable length to allow for the preparation of a thorough RFP.
In addition, there may be an opportunity for an ‘out of cycle’ request for research funding that will be considered independently and at the discretion of the ANA leadership. Information on the timelines of the grant cycle will be made available through select ANA publication, digital, and communications platforms.
For further information, please contact Jim Shea, CEO, at jimshea@anausa.org.
Proposal Guidelines
Our Research Task Force has established strong guidelines for those applying for a grant. Included within these guidelines (five-page limit for proposal) are requests for:
- Summary of most recent/relevant research and a statement of primary purpose
- A concise Statement of Goals
- An Impact Statement
- Research Strategy including Participants, Study Design, Analysis Method, and Potential Outcomes
- Budget, including a statement that the ANA does not provide funding for indirect costs
- Project Timeframe
- List of Personnel Support
Proposal detail requirements can be found here, and helpful applicant guidelines can be found here. Please submit all proposals to researchgrantprogram@anausa.org.
Award Review Process
All grant submissions are reviewed by our Research Committee and reviewing parties include ANA Board members and third-party scientists with specialization in the area of acoustic neuroma. All grant submissions are considered using a blind review process and a funding recommendation will be confirmed by a decision of the ANA Board of Directors.
Schedule for upcoming cycle:
- Request for Proposals - TBD
- Full Proposal deadline - TBD
- Review of Proposals - TBD
- Announcement of Grant Funding - TBD



























































