- Details
ANA Virtual Patient Education Event
Hosted by The Ohio State University Wexner Medical Center
January 24, 2026
12:00 - 5:00 pm ET

Scheduled presenters:


Oliver Adunka, MD
Dr. Adunka is an otolaryngologist with a specialization in skull base and neurotologic surgery. He is dedicated to advancing surgical techniques for the treatment of vestibular schwannoma and improving patient outcomes through multidisciplinary collaboration.


Meghan Hiss, AuD
Meghan Hiss is an audiologist specializing in hearing rehabilitation and auditory disorders. She is committed to improving patient outcomes through advanced hearing technologies and personalized care.


Elise Dole, PT, DPT
Elise Dole is a physical therapist with expertise in vestibular rehabilitation and neuro-oncology care. She focuses on helping patients regain balance and mobility following treatment for vestibular schwannoma.


Steven Ciolek, PT, DPT
Steven Ciolek is a physical therapist specializing in neurological rehabilitation, particularly for patients affected by acoustic neuroma.


Desi Schoo, MD
Dr. Schoo is a cranial base surgeon who specializes in hearing loss, balance disorders, chronic middle ear disease, acute vestibular dysfunction, and skull base tumors.


Robert Macielak, MD
Dr. Macielak is a neurosurgeon specializing in skull base surgery and complex tumors, including vestibular schwannoma. He integrates patient preferences and clinical data to guide individualized surgical decisions.


Kyle Wu, MD
Dr. Wu is an otolaryngologist and neurotologist focused on the surgical management of vestibular schwannoma.


Daniel Prevedello, MD
Dr. Prevedello is a Professor of Neurosurgery and Otolaryngology. He serves as Co‑Director of the Comprehensive Skull Base and Pituitary Surgery Center and Director of the Minimally Invasive Cranial Surgery Program. Dr. Prevedello is also Co‑Director of the Anatomy Laboratory Toward Visuospatial Surgical Innovations in Otolaryngology and Neurosurgery (ALT‑VISION)
- Details

ANA Patient Education Event
Hosted by
Saturday, March 7, 2026
7:30 am - 3:30 pm ET
Join us for our upcoming Patient Education Event at Mayo Clinic’s beautiful Jacksonville, Florida campus on Saturday, March 7.
This free event is hosted by Mayo Clinic Florida’s Acoustic Neuroma Program, which specializes in the treatment of acoustic neuromas. Mayo Clinic experts use innovative, patient-centered techniques to achieve exceptional outcomes.
Hear directly from Mayo Clinic’s integrated care team – including neurotologists, neurosurgeons, neurologists, facial plastic surgeons, and audiologists – as they share the latest advances in treatment decision-making, recovery, and patient experience.
Whether you’re newly diagnosed or already navigating your care and exploring treatment options, you’ll find valuable information, inspiration, and connection at this event. Don’t miss this opportunity to learn, connect, and engage with experts in acoustic neuroma treatment!
|
Scheduled Topics Include: |
Scheduled Presenters
Joseph Breen, MD


Joseph Breen, M.D., serves as chief of the Division of Otology and Neurotology at Mayo Clinic in Florida. As an expert ear surgeon, Dr. Breen specializes in the diagnosis and treatment of acoustic neuromas (vestibular schwannomas), hearing loss, chronic ear infections and rare or complex ear disorders.
Richard Byrne, MD


Richard Byrne, M.D., is the Chair of Neurosurgery at Mayo Clinic in Florida. He was recently named President of the Society of Neurological Surgeons and has served in leadership roles in numerous professional organizations. Dr. Byrne has 25 years' surgical experience and expertise in all types of brain tumor surgery, including acoustic neuromas.
Mallory Raymond, MD


Mallory Raymond, M.D., is a fellowship-trained Otologist/Neurotologist and Assistant Professor of Otolaryngology–Head and Neck Surgery at Mayo Clinic in Jacksonville, Florida. She specializes in advanced surgical care for outer, middle, and inner ear disorders, with expertise in the management of complex hearing and balance conditions. Dr. Raymond is dedicated to delivering leading-edge, patient-centered care for individuals with acoustic neuromas and other skull base disorders.
Jennifer Peterson, MD


Jennifer Peterson. M.D., is an Associate Professor in Radiation Oncology with a joint appointment in Neurosurgery at Mayo Clinic in Florida. She leads clinical research into CNS tumors—including acoustic neuroma—focusing on optimizing radiotherapy strategies that balance efficacy with minimizing toxicity.
Alfredo Quinones-Hinojosa, MD


Alfredo Quinones-Hinojosa, M.D., is a neurosurgeon and former chair of the Department of Neurologic Surgery at Mayo Clinic in Florida, where he also held the Monica Jacoby Chair and the William J. and Charles H. Mayo Professorship. His clinical and research work focuses on advanced surgical treatment of brain and spinal tumors, functional mapping, minimally invasive skull base approaches, and NIH-funded research aimed at curing brain cancer.
Ninoska Alvarez, PT, MSPT


Ninoska Alvarez, P.T., MSPT, serves as lead physical therapist at the Brooks Balance Center in Jacksonville, Florida. A vestibular-certified therapist since 2006, she has focused her practice on treating vestibular disorders for nearly 20 years across both inpatient and outpatient settings. She has worked at the Brooks Balance Center—northeast Florida’s only specialty center for vestibular disorders—since 2008.
Deanna Menapace, MD


Deanna Menapace, M.D., is the Chief of the Division of Facial Plastic & Reconstructive Surgery in the Department of Otolaryngology at Mayo Clinic in Florida. Her clinical expertise includes the management of facial nerve dysfunction, with a focus on both cosmetic and functional interventions to improve facial comfort, quality of life and restore facial symmetry.
Greta Stamper, AuD, PhD


Greta Stamper, Au.D., Ph.D., is a clinical and research audiologist in the Department of Otolaryngology at Mayo Clinic in Florida. She serves as chief of the Division of Audiology and leads adult aural rehabilitation and hearing-loss prevention efforts.
Joseph Breen, MD


Joseph Breen, M.D., serves as chief of the Division of Otology and Neurotology at Mayo Clinic in Florida. As an expert ear surgeon, Dr. Breen specializes in the diagnosis and treatment of acoustic neuromas (vestibular schwannomas), hearing loss, chronic ear infections and rare or complex ear disorders.
Richard Byrne, MD


Richard Byrne, M.D., is the Chair of Neurosurgery at Mayo Clinic in Florida. He was recently named President of the Society of Neurological Surgeons and has served in leadership roles in numerous professional organizations. Dr. Byrne has 25 years' surgical experience and expertise in all types of brain tumor surgery, including acoustic neuromas.
Mallory Raymond, MD


Mallory Raymond, M.D., is a fellowship-trained Otologist/Neurotologist and Assistant Professor of Otolaryngology–Head and Neck Surgery at Mayo Clinic in Jacksonville, Florida. She specializes in advanced surgical care for outer, middle, and inner ear disorders, with expertise in the management of complex hearing and balance conditions. Dr. Raymond is dedicated to delivering leading-edge, patient-centered care for individuals with acoustic neuromas and other skull base disorders.
Jennifer Peterson, MD


Jennifer Peterson. M.D., is an Associate Professor in Radiation Oncology with a joint appointment in Neurosurgery at Mayo Clinic in Florida. She leads clinical research into CNS tumors—including acoustic neuroma—focusing on optimizing radiotherapy strategies that balance efficacy with minimizing toxicity.
Alfredo Quinones-Hinojosa, MD


Alfredo Quinones-Hinojosa, M.D., is a neurosurgeon and former chair of the Department of Neurologic Surgery at Mayo Clinic in Florida, where he also held the Monica Jacoby Chair and the William J. and Charles H. Mayo Professorship. His clinical and research work focuses on advanced surgical treatment of brain and spinal tumors, functional mapping, minimally invasive skull base approaches, and NIH-funded research aimed at curing brain cancer.
Ninoska Alvarez, PT, MSPT


Ninoska Alvarez, P.T., MSPT, serves as lead physical therapist at the Brooks Balance Center in Jacksonville, Florida. A vestibular-certified therapist since 2006, she has focused her practice on treating vestibular disorders for nearly 20 years across both inpatient and outpatient settings. She has worked at the Brooks Balance Center—northeast Florida’s only specialty center for vestibular disorders—since 2008.
Deanna Menapace, MD


Deanna Menapace, M.D., is the Chief of the Division of Facial Plastic & Reconstructive Surgery in the Department of Otolaryngology at Mayo Clinic in Florida. Her clinical expertise includes the management of facial nerve dysfunction, with a focus on both cosmetic and functional interventions to improve facial comfort, quality of life and restore facial symmetry.
Greta Stamper, AuD, PhD


Greta Stamper, Au.D., Ph.D., is a clinical and research audiologist in the Department of Otolaryngology at Mayo Clinic in Florida. She serves as chief of the Division of Audiology and leads adult aural rehabilitation and hearing-loss prevention efforts.
- Details
ANAwareness Week 2025
June 8 - 14
Informed Decisions - Better Outcomes
All available recordings of our ANAwareness Week presentations
are now available in our video library and our member webinar library.
(click each picture to expand)
Chuck


Acoustic Neuroma tattoos...ANA is Great !!
Jennifer


AN Warrior!(Translabyrinthine surgery March 2021)
Brenda


13 years since acoustic neuroma surgery! I'm thankful and blessed to make it through the struggles and accepting my new normal! Face numbness and loss of hearing remain, headaches have diminished over time! Praise God!
Megan


I had a 3.5cm acoustic neuroma surgically removed on 6/26/23. Here is a photo of our family vacation last week hiking in Banff, Alberta. We live in Austin, TX so the break from the heat was fantastic. What was even better was I was able to hike with my husband and kids again with balance and strength I haven’t had since pre-surgery. It was a long road to recovery but life is good! (That’s me on the left).
Yvette


Just celebrated my 7th ANniversary from retrosigmoid surgery and 10 years since diagnosis. I am so grateful for my successful outcome and my strength to keep pushing past the balance issues, headaches, hearing loss, etc. and enjoy this life!
Emily


My daughter and I celebrated Acoustic Neuroma Awareness Week and my 10th ANniversary raising money for the Acoustic Neuroma Association at the Walk4Hearing.
Bonny


After AN surgery in 2017, I am happy to continue my part-time job as Choir Director and Organist.
Enrica


My daughter and I nine months post op from the translabyrinthine surgery. I am working on balance and dealing with delayed wound healing & headaches, but getting better!
Vivian


Diagnosed 7 years ago, had gamma knife radiation May 2025. Hope it works 🤞 Vivian Canada 🇨🇦
Mary


Mary – a little over a year after surgery. Not fast, but truly thankful
Melony


My name is Melony and I’m a 25 year old actor from Chicago, Illinois. I was diagnosed with a 4.5cm Unilateral Vestibular Schwannoma. On April 9th, 2025, I underwent a 13 hour surgery and I am currently 10 weeks post op. Big thanks to Dr. Stephen Magill, Dr. Kevin Zhan, and the team at Northwestern Memorial Hospital.
Kathy


Kathy and Grandson Elijah
Amy
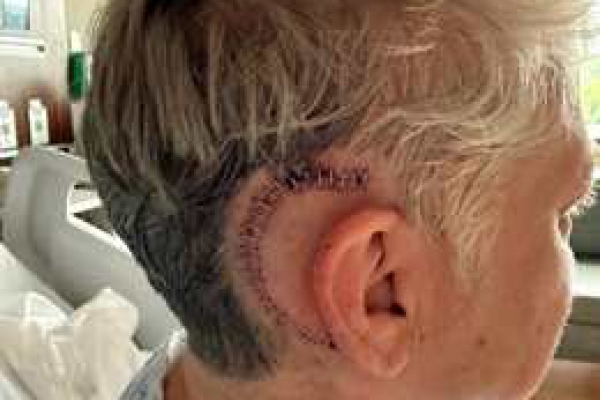
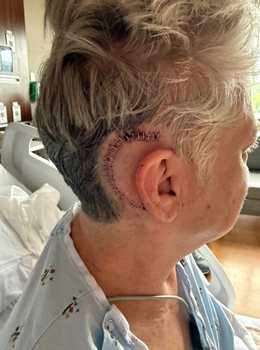
Perfect week to have mine removed.😊 This photo is from 6/14/25, two days post surgery.
Alexandra


5 years post-op (3cm, translab). I celebrated by running my 5th half marathon and I'll be running my 5th marathon this October!
Laura


At the Tulip Festival in Fayetteville, Arkansas.
Brooke


2.5 years strong #ANwarrior
James
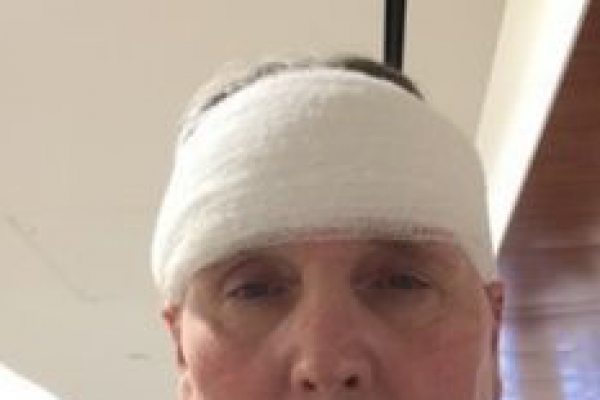
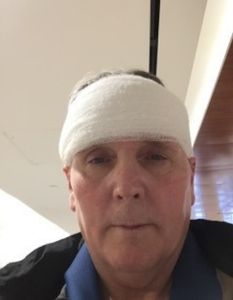
I am a 3-time AN warrior. Yes, 3 times the AN has grown back, and 2 brain surgeries.
Thank you to our presenting sponsor, Lilly,
and all of our sponsors for partnering with us.















































































